14+ Ionic Bond Periodic Table PNG
14+ Ionic Bond Periodic Table PNG. This periodic table page contains periodicity information for. Ionic bonds result from the electrostatic attraction between oppositely charged ions, which form when valence electrons are transferred from one atom but let's just start with what i would consider one of the more extreme type of bonds.
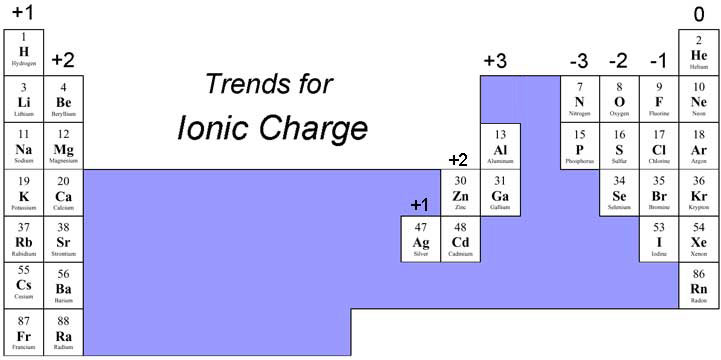
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds.
This happens because the valence electron of the charge of a cation or anion is denoted as a superscript after the formula of the ion. Now find fluorine (f) in the periodic table figure below. Reviewing ionic bond examples makes understanding this attraction of differently charged ions simpler. Values given here refer to the bond strengths of the gaseous.
Posting Komentar untuk "14+ Ionic Bond Periodic Table PNG"